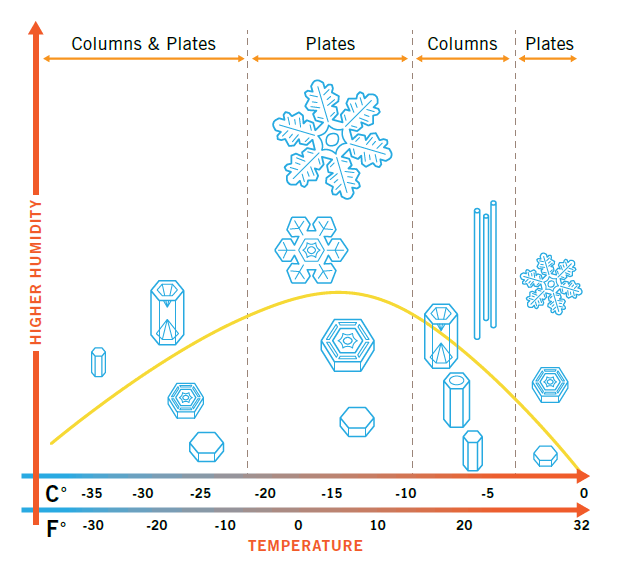
by Dr. Larry Vardiman, Answers Magazine, 12/27/15Used with permission. How can one type of crystal adopt so many beautiful forms? Some of the world’s greatest scientific minds have puzzled over this question. Cloaked in the mid-winter darkness, John quickly made his way across the Charles Bridge to the newer residential section of town. His destination was on the opposite side of the river from downtown Prague and the castle. He was running late for the New Year’s Eve party. To make things worse, the shops were closed where he might have purchased a gift for the host, his patron who had paid for his scientific research over the past year. As he passed under one of the lamps on the bridge, he noticed snow had begun falling lightly. Small, individual snow crystals were collecting on the dark fleece of his jacket. He stopped abruptly and watched with fascination as one geometric shape after another fell onto the arm of his coat. Their designs glowed brilliantly in the flickering light from the lamp above. Here was a small hexagon; there a featherlike pattern; a third, the shape of a star. Yet each crystal had six corners. Why Are Snow Crystals So Varied? When water freezes, it forms a simple, solid, six-sided crystal. As each crystal falls through the cloud, the crystal grows. Depending on the water in the air (humidity) and the temperature, the shape and direction varies. While the possibilities are infinite, two basic shapes are most common: columns and plates. The large, branched stars (the most familiar type of plate) require both high humidity and a very specific cold temperature (around 5°F, or –15°C).
Never before had John taken the time to investigate individual snow crystals. Normally in snowstorms a dozen or more crystals would fall jumbled together in a large snowflake so he couldn’t discern the individual crystals. But tonight they fell individually. The air was probably so cold and the snow falling so lightly that they didn’t clump together. As John studied the various shapes and sizes, an idea began to form—he would explain why snow crystals all had six corners and present his thoughts to his patron as a gift! He began to speculate. What if snow crystals are built from small particles, so small that they can’t be seen with the naked eye? Could these particles fit together in a pattern to form hexagonal shapes with six corners? He didn’t just think in two dimensions. How could particles fill three-dimensional space to form hexagonal shapes? He visualized a stack of cannon balls and realized, when viewed from above, the stack forms a hexagonal pattern. He considered why bees construct hexagonally shaped honeycombs and why flowers have different numbers of petals. He considered how pomegranate seeds fill space and other objects form hexagonal patterns, always with the question in mind—“Why do snow crystals have six corners?” Today John is known as Johannes Kepler, the famous German astronomer who later developed the laws for elliptical orbits of planets. His laws formed the basis for Newton’s law of gravity. He didn’t have microscopes to validate some of his ideas. But even today with high-power microscopes, we don’t have a complete explanation for how snow crystals grow. It’s a source of endless fascination. Because of the pamphlet he later presented to his patron in 1611 (On the Six-Cornered Snowflake), he is recognized by many today as the father of crystallography. He was unable to prove to his own satisfaction that the packing of small particles adequately explained six-cornered snow crystals, but he was on the right track. His insightful treatise shows how the relatively new scientific method combined careful observation and testing theories. In addition to his proposal that particles fill space to form snow crystals, Kepler suggested that the Creator instituted a built-in “formative principle” which determines the shape. We know today that information is built into water molecules, which causes them to form hexagonal shapes when they freeze together as snow crystals. If the humidity is too dry or the temperature too warm, the crystal will remain solid without branching patterns. Despite centuries of study, scientists still haven’t figured out all the laws of water crystal growth. Today crystallographers have classified over 80 basic types of snow crystals. Some patterns are even more complicated than six corners. The basic hexagonal pattern can be modified in a number of ways as the cloud’s temperature and moisture varies. Snow crystals grow in one dimension faster than in the other, depending on the temperature. As humidity increases, the corners develop more branches. Kepler’s argument for a “formative principle” is still valid today, not just for snow crystals but for all types of molecular processes. These are our God-given laws of chemistry and physics. The marvelous rules that govern such processes do not produce “organized complexity.” Molecules like DNA are examples of organized complexity because they use a language and store information to be retrieved later. Snowflakes are complex but not “organized” in this sense. So we can’t argue that the beautiful designs of snowflakes disprove evolution. What does contradict evolution is the existence of the unchanging chemical and physical laws, attributes that our Creator God built into the molecules from the beginning of creation. “Since the creation of the world His invisible attributes are clearly seen, being understood by the things that are made, even His eternal power and Godhead so that they are without excuse” (Romans 1:20). The next time a snow crystal lands on your sleeve, take the time to enjoy its beauty, and share the glories of its Designer with the next person you meet at the party you’re headed to! Dr. Larry Vardiman is currently serving as a professor of science and math for Shasta Bible College& Graduate School in Redding, CA. In the past he has served as professor of atmospheric science and chair of the Department of Astro-geophysics for the Institute for Creation Research until his retirement in 2012. He holds a bachelor’s degree in physics and meteorology and an MS and PhD in atmospheric science Comments are closed.
|
|